Epigenetics
We all know that our surroundings and life experiences strongly affect who we are. But did you know these same circumstances change our bodies at the cellular level? The underlying cellular mechanism for the ability of our environment and experiences to affect our bodies is called epigenetics.
While most people are familiar with the inheritance of genes from one generation to the next, the activity of genes (or gene expression) is actually determined by epigenetics, which literally means “on top of” genetics. DNA contains the instructions that tell our cells how to make proteins; an important task given proteins do almost all the work in our bodies. The instructions in DNA are identical in every cell in the body and do not change with time.
However, our cells do not make the exact same proteins all the time, nor do they make them in the exact same amounts. Epigenetics is a way for the DNA’s instructions to be read differently – in response to our environment, experiences, and health – leading to changes in gene expression, which then result in changes in the proteins made by that cell.
We can think of cells as an assembly line: the DNA contains the templates (genes) for all the components (proteins) that need to be assembled, while the foreman (epigenetics) instructs the line on which components are needed, and in what quantities.
Breaking Down a Complex Process
Our chromosomes, inherited from our parents, are made up of DNA wound tightly around proteins, bundled together in an elegant hierarchical structure.
Tightly-coiled chromatin fibers are made up of smaller units (nucleosomes) which contain individual DNA strands wrapped around specialized proteins (histones). Part of the histone protein – known as the tail – protrude from the nucleosome and can be “marked”.
This is where the process of changing the DNA’s instructions begins; several different cellular components can interact with these marks, resulting in gene expression being turned on, off, up or down. These cellular components can be described as “writers” or “erasers” that can change the epigenetic message by adding or removing marks. Another type, “readers”, can interpret the message by reading marks on the histone tails.
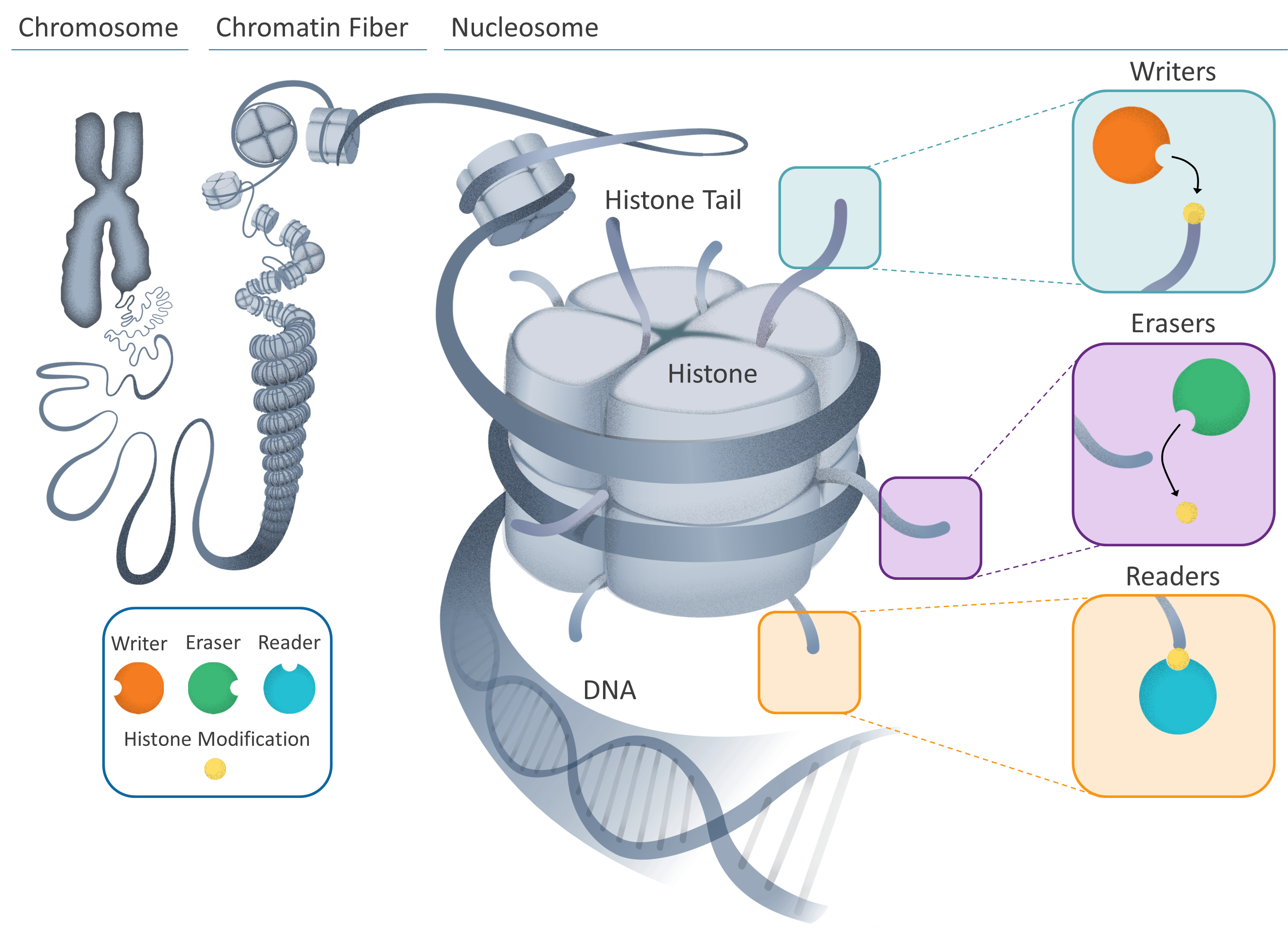
Bromodomain and extraterminal domain (BET) proteins are one type of reader, which bind to the marks on histone tails, read the new epigenetic message and recruit other cellular components to begin the process of making proteins.
Through years of research, we know that BET proteins are involved in reading disease-related epigenetic marks, resulting in the production of harmful proteins which can have wide-ranging negative effects on the body. Our ongoing work focuses on the regulation of gene expression through the inhibition of BET proteins, thereby restoring biological functions – altered by serious illnesses such as cardiovascular disease and others – to a healthy state.
