Clinical
Resverlogix is committed to developing epigenetic therapies for the benefit of patients with chronic disease. Our current clinical program focuses on advancing our lead compound – apabetalone – and evaluating its therapeutic potential in the treatment of vascular disease and Post COVID-19 Conditions.
clinical Indications
Through its unique, epigenetic mechanism, apabetalone acts to normalize the expression of key proteins and pathways that are dysregulated by cardiovascular disease. Clinical data has shown this treatment translates to direct benefits to patients, including observed reductions in major adverse cardiac events (MACE).
The Phase 3 Study, BETonMACE, found apabetalone treatment results in a 41% hazard reduction in first hospitalizations due to heart failure (HHF), when compared to a placebo with top standard of care. A hazard reduction of 23% was found when HHF and MACE were combined.
A recent estimate from the US Centers for Disease Control and Prevention (CDC) suggests that as many as one-in-three US adults may experience Post COVID-19 Conditions (PCC), colloquially known as long-COVID, after contracting the viral illness. Apabetalone has the potential to treat and prevent PCC by reducing the overactive inflammatory response that is associated with negative outcomes in the weeks and months following initial infections.
A Phase 2/3 clinical study to assess the safety and efficacy of apabetalone in the treatment of PCC is currently underway.
There is a direct link between heart disease, diabetes and chronic kidney disease, with high blood pressure and diabetes as the main causes of chronic kidney disease. Findings from BETonMACE demonstrated a significant reduction in MACE (hazard reduction 50%) with apabetalone treatment among the CKD subpopulation.
Apabetalone continues to be evaluated for the treatment of patients with chronic kidney disease. A Phase 2A program is also planned for CKD patients on dialysis, which will examine apabetalone’s safety in this population and as well as its ability to improve key biomarkers.
Both diabetes and CVD have been associated with higher levels of dementia and neurocognitive problems. Moreover, multiple proteomics assessments have revealed significant overlap between processes associated with CVD – vascular inflammation and calcification – and the biological pathways that drive cognitive risk. Our Phase 3 trial, BETonMACE, included a pre-specified analysis of cognition scores in patients over the age of 70, utilizing the MoCA assessment. In patients with a baseline MoCA <22, apabetalone treatment was associated with significant MoCA score improvements versus placebo. There were additional significant improvements with apabetalone in biomarkers associated with moderate to severe cognitive decline and dementia including ALP.
Due to the positive effects of apabetalone treatment on primary lung smooth muscle cells (SMCs) and in animal models, an investigator-initiated pilot study of the effect of apabetalone on top of standard of care in pulmonary arterial hypertension is underway.
Clinical Trial History of apabetalone
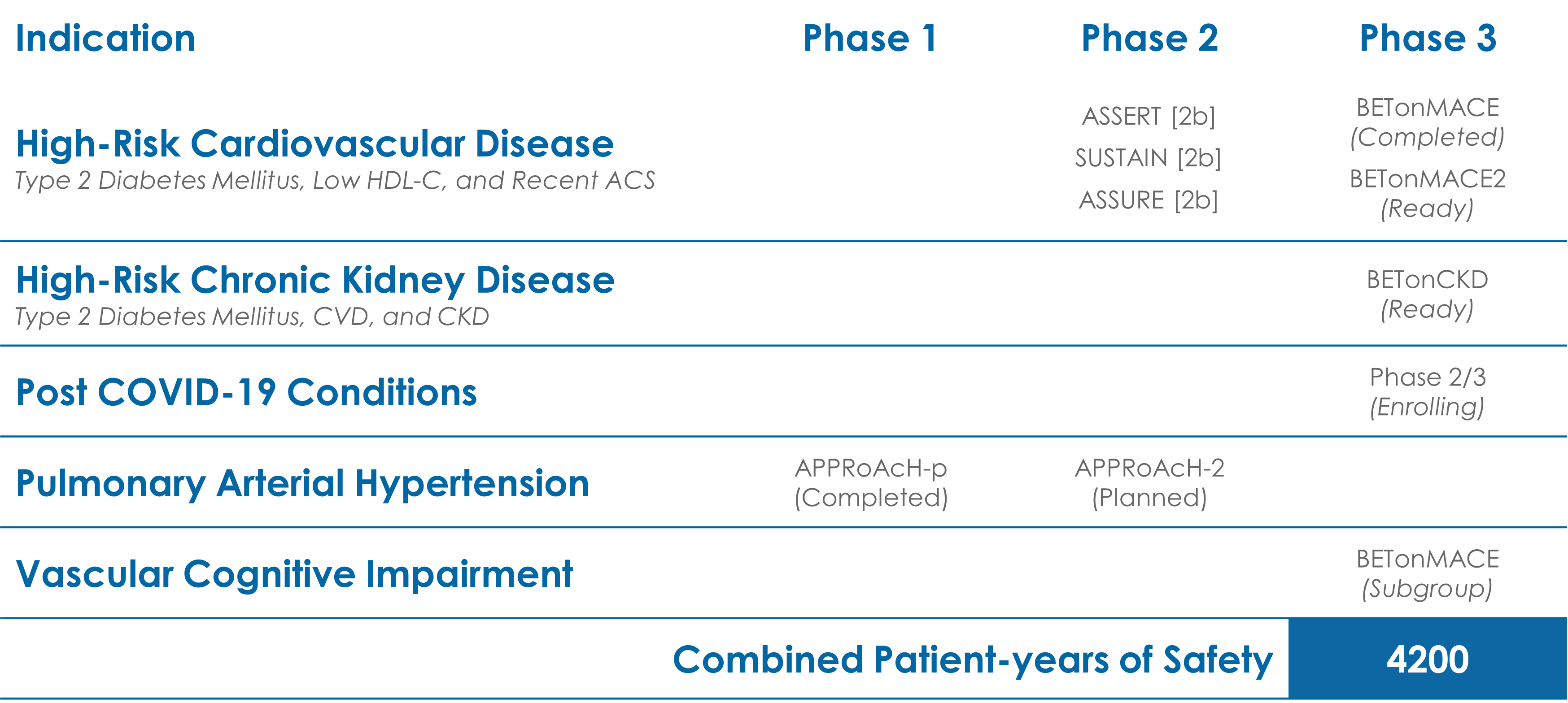
For more information on the groundbreaking Phase 3 trial, BETonMACE, click HERE.
Clinical Steering & Advisory Boards
Our appointed committee members hold the highest levels of expertise in a multitude of medical fields.
Clinical Steering Committee (CSC)
BSc (hons), MBChB, MD, MPhil (Cantab), FACC, FAHA, FESC, FRCP
Chairman
Professor Ray’s research interests focus on the prevention of cardiovascular disease using observational methods and intervention studies including large trials.
- Professor of Public Heath, Department of Primary Care and Public Health, School of Public Health, Imperial College London, UK
- Established the Familial Hypercholesterolaemia (FH) Studies Collaboration (FHSC)
- Principal Investigator for the TOGETHER study
MD, FAHA
Member
Dr. Ginsberg’s research focuses on the regulation of lipoprotein metabolism, particularly in relation to insulin resistance and diabetes.
- Irving Professor of Medicine at Columbia University College of Physicians and Surgeons
- Associate Dean for Clinical and Translational Research
- Director of the Irving Institute for Clinical and Translational Research at Columbia University Medical Center
- Principal Investigator in the landmark ACCORD Lipid Trial
MD, MPH, PhD, FAAP, FACP, FASN, FAHA, FNKF
Member
Dr. Kalantar’s research focuses on kidney disease outcomes and cardiovascular risks in CKD and diabetes. Dr. Kalantar has authored or coauthored over 400 peer reviewed research articles and scientific papers and 15 book chapters.
- Professor of Medicine, Pediatrics, Public Health and Epidemiology
- Chief of the Division of Nephrology and Hypertension at UC Irvine School of Medicine
- President Elect of the International Society of Renal Nutrition and Metabolism
- Associate Editor of several journals including the American Journal of Kidney Diseases and Nephrology Dialysis Transplantation
MBBS, PhD
Member
- Professor of Cardiology at the University of Adelaide
- Deputy Director at the South Australian Health and Medical Research Institute
- Principal Investigator of the SATURN, AQUARIUS, ACCELERATE, STRENGTH and GLAGOV trials
- Fellow of the Royal Australasian College of Physicians and American College of Cardiology
- Member of the American Heart Association
MD, PhD
Member
- Professor of Medicine in the Division of Cardiology of the University of Colorado Denver
- Lead investigator in MIRACL (atorvastatin), dAL-OUTCOMES (dalcetrapib), and ODYSSEY Outcomes (alirocumab) trials
- Fellow of the American Heart Association and the American College of Cardiology
- Member of the American Physiological Society and the International Society for Heart Research
MD, PhD, FAAFP, FICA, FAHA, FNLA, FCCP, FACC
Member
- Chief of Medicine for CGH Medical Center
- Clinical Professor at the University of Illinois School of Medicine
- Associate Professor of Medicine at Johns Hopkins University School of Medicine
- Member of the American College of Cardiology Foundation Council on Cardiovascular Disease Prevention
- Member of the American Heart Association’s Council on Lipoproteins, Lipid Metabolism, and Thrombosis
Renal Clinical Advisory Board (RCAB)
MD, MPH, PhD
Chairman
- Professor and Chief, Division of Nephrology and Hypertension at University of California, Irvine
- Founder and Director of the Harold Simmons Center for Kidney Disease Research and Epidemiology
- Associate Editor of several peer-reviewed journals including Nephrology Dialysis Transplantation (NDT), American Journal of Kidney Diseases (AJKD), Cardiorenal Medicine (CRM), Seminars in Dialysis, Journal of Cachexia, Sarcopenia and Muscle (JCSM)
- Member of the editorial board of Kidney International (KI), Journal of American Society Nephrology (JASN), Nature Reviews Nephrology, American Journal of Nephrology (AJN)
MD
Member
Dr. Beddhu’s clinical and research interests include hypertension, chronic kidney disease progression and complications and end-stage renal disease.
- Professor of Medicine at the University of Utah School of Medicine
- Board Certified in Internal Medicine and Nephrology
- Served on several national committees including NIH panels, American Society of Nephrology Research Committee and NKF Clinical Practice Guidelines Committee
MD
Member
Dr. Brandenburg's primary focus is on chronic kidney disease – mineral and bone disorder, cardiorenal syndrome, and calciphylaxis.
- Nephrologist, Associate Professor and Senior Consultant at the Department of Cardiology, Intensive Care Medicine and Vascular Medicine, University Hospital of the RWTH Aachen, Germany
- Leader of the German Calciphylaxis Registry
- Board member of the ERA-EDTA Scientific Working Group Chronic Kidney Disease – Mineral and Bone Disorder (CKD-MBD)
- Member of the German and European Societies of Nephrology and the Societies of Cardiology
MD, PhD
Member
Dr. Haarhaus’s research at the Division of Renal Medicine, Karolinska Institutet, focuses on the link between skeletal disorders and cardiovascular complications in chronic kidney disease, with a special focus on alkaline phosphatase.
- Consultant Nephrologist at the Department of Nephrology, Karolinska University Hospital, Stockholm, Sweden where he is head of the Bone and Mineral Program
- Member of the Chronic Kidney Disease – Mineral and Bone Disorder (CKD-MBD) Working Group of the European Renal Association – European Dialysis and Transplantation Association (ERA-EDTA)
- Member of the Guidelines Committee of the Swedish Society of Nephrology
MD, SM, FRCPC
Member
Dr. Tonelli was named a “Highly Cited” researcher in 2015 by Thomson-Reuters, corresponding to a rank in the top 1% by citations of all researchers worldwide for field and publication year.
- Associate Vice-President (Research) at the University of Calgary
- Fellow of the Canadian Academy of Health Sciences
- Member of the American Society for Clinical Investigation in 2014
MD, FASN, FNKF, FERA
Member
Dr. Zoccali is a specialist in Renal Diseases (Pisa University) and Hypertension.
- Director, Division of Nephrology, Hypertension and Renal Transplantation, Ospedali Riuniti, Reggio Cal, Italy
- Chief, CNR-IBIM Clinical Epidemiology and Pathophysiology of Renal Diseases and Hypertension
- Professor, Postgraduate Schools of Nephrology, Palermo, Catania and Messina Universities
- Editor in Chief, Nephrology Dialysis and Transplantation
- Academic Editor, (Nephrology) PlosOne
- Editorial Board member, Journal of the American Society of Nephrology (JASN)
- Editorial Board member, Clinical Journal of the American Society of Nephrology (cJASN)
- Editorial Board member, Kidney International (KI)
Neurodegenerative Clinical & Scientific Advisory Board (NCSAB)
MD, PhD
Chairman
Dr. Winblad’s research interests focus on the basic mechanisms behind, as well as treatment of, Alzheimer’s disease.
- Professor at the Karolinska Institutet Alzheimer Disease Research Center in Huddinge, Sweden
- Chief Physician at Karolinska University in Huddinge
- Guest professor at the Department of Psychiatry in Frankfurt
- Honorary professor at Beijing University, Wuhan University and Shanghai University in China
- Member of the Advisory Committee for the Medical Research Council
- Co-chair of the European Alzheimer’s Disease Consortium (EADC)
- Chair of the Medical Scientific Advisory Panel of Alzheimer’s Disease International (ADI)
MD, ScD
Member
Dr. Cummings is an experienced clinical trialist with expertise in clinical trial design and analysis, global trial implementation, and trial outcome measures.
- Director of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas, Nevada and Cleveland, Ohio
- Camille and Larry Ruvo Chair of the Neurological Institute of Cleveland Clinic
- Author of the Neuropsychiatric Inventory (NPI)
- Former Professor of Neurology and Psychiatry at UCLA, Director of the Mary S. Easton Center for Alzheimer’s Disease Research at UCLA, and Director of the Deane F. Johnson Center for Neurotherapeutics at UCLA
MD, PhD
Member
Dr. Koldamova’s current research is concentrated on the role of lipoproteins in AD pathogenesis using transgenic mice expressing human ApoE isoforms.
- Associate Professor at the University of Pittsburgh, Pittsburgh, PA
MD, PhD
Member
Dr. Zetterberg is has spent the last 10 years focusing on the development of biomarkers for Alzheimer’s disease and other brain disorders.
- Developed new diagnostic tests for Alzheimer’s disease, as well as new preclinical models
- Professor of neurochemistry at the University of Gothenburg
